Transforming Care Through Actionable Biomarkers
BioPorto is dedicated to providing clinicians with tools to make impactful changes in patient management; currently, the NGAL biomarker to aid in the risk assessment and diagnosis of acute kidney injury.
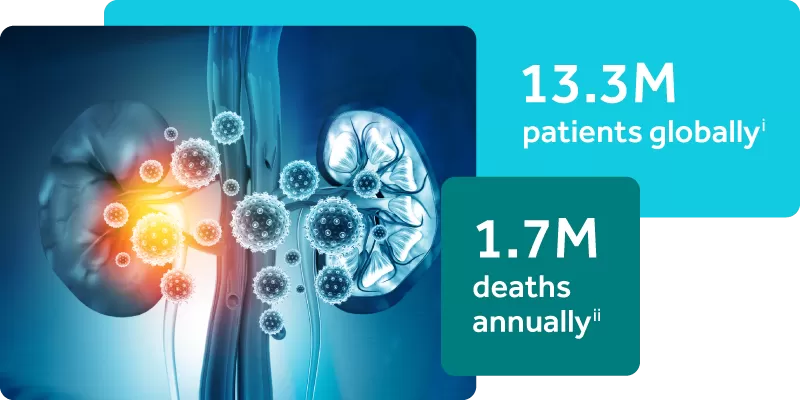
Acute Kidney Injury is a Silent Epidemic
factors such as nutritional status and muscle mass.iii
Empowering Early Intervention
Rapidly detect acute kidney injury with The NGAL Test™. Unlike traditional methods, an NGAL result identifies kidney damage early, guiding interventions with the potential to prevent irreversible damage. Take confident action now.
*The NGAL Test is CE-marked and available for in vitro diagnostic use in the EU. It is registered in Canada, Korea, Israel and other countries.

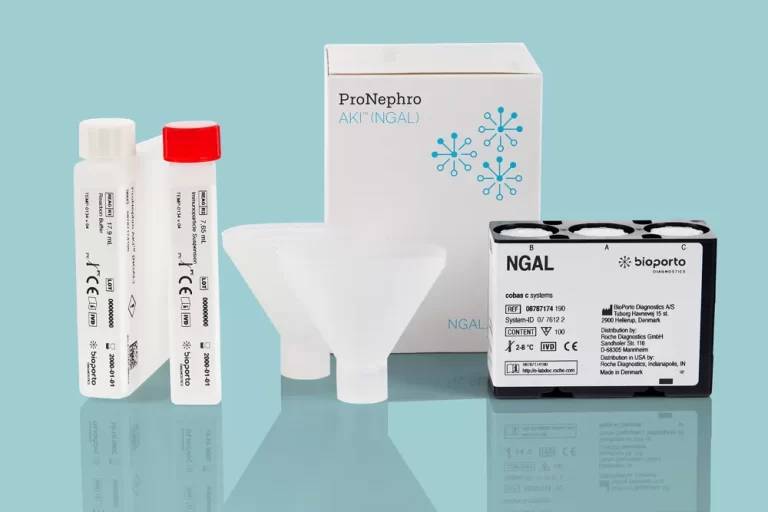
Revolutionizing Pediatric AKI Risk Assessment
BioPorto welcomes ProNephro AKI (NGAL) to its family of NGAL products in the race against kidney damage – the first FDA-cleared biomarker for pediatric acute kidney injury risk assessment. US commercial availability through Roche Diagnostics is expected in 2024.
*ProNephro AKI is FDA-cleared for use in the US. Commercial availability expected in 2024.
Supporting Research
BioPorto offers a unique range of human and animal NGAL ELISA kits for preclinical animal research and Phase 1 human clinical trials.
Join us in changing the kidney care paradigm
Stay up to date with the latest resources for kidney care, and news about BioPorto.
Additional Resources
iMehta RL, Burdmann EA, Cerdá J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. 2016. Lancet. 2016;387(10032):2017-2025.
iiLewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84(3):457-467.
iiiMoledina DG, Parikh CR. Phenotyping of Acute Kidney Injury: Beyond Serum Creatinine. Semin Nephrol. 2018;38(1):3–11.
